姜 帅 | 博士 | 教 授 | 博士生导师 | ||
科 室: | 药剂学实验室 | ||||
电子邮箱: | jiangshuai@ouc.edu.cn |
|
| ||
研究方向: | 1. 纳米-生物相互作用:作用机制、生物效应与调控策略 2. 纳米药物蛋白冠的分析检测技术及其生物效应研究 3. 生物大分子递送体系及其在药物递送和合成生物学中的应用 | ||||
个人简介 |
|
|
|
| |
教授,博士生导师。2016年于中国科学院获博士学位,先后于德国马普高分子所进行博士联培、博士后研究、担任课题组长。2021年加入海大医药学院,从事纳米药物递送系统与纳米-生物界面作用的研究工作。主持国自然青年基金、山东省优秀青年基金(海外)、“泰山学者”青年专家等项目。发表SCI论文50余篇,其中以第一或通讯作者在Chem. Soc. Rev.、Acc. Chem. Res.、Sci. Adv.、Angew. Chem.(6)、Adv. Mater.、Adv. Funct. Mater.、Adv. Sci.、ACS Nano、Nano Lett.(2)等期刊发表论文30余篇,引用2300余次。
Google Scholar: https://scholar.google.de/citations?user=QU1qNosAAAAJ&hl=en Researchgate: https://www.researchgate.net/profile/Shuai-Jiang-10
| |||||
教育背景 |
|
|
|
| |
2013.12~2015.11 | 德国马普学会高分子研究所 | 高分子物理化学 | 博士联培 | ||
2011.09~2016.06 | 中国科学院大学 山西煤炭化学研究所 | 工业催化 | 硕博连读 | ||
2007.09~2011.06 | 烟台大学 | 化学工程与工艺 | 学士 | ||
工作经历 |
|
|
|
| |
2021.05~至今 | 中国海洋大学医药学院 | 教授 |
| ||
2019.09~2021.04 | 德国马普学会高分子所 | 课题组长 |
| ||
2016.08~2019.08 | 德国马普学会高分子所 | 博士后 |
| ||
|
|
|
| ||
研究进展 |
|
|
|
| |
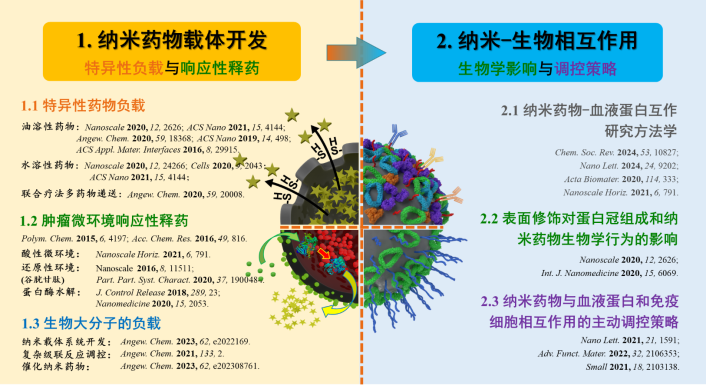
纳米药物靶向递释系统是一种极具开发潜力的药物递送策略,其通过改变药物的代谢动力学和组织分布,提高对肿瘤组织的特异性作用并同时降低药物对正常组织的毒性,在肿瘤治疗等领域得到了广泛的重视和发展。针对现阶段抗肿瘤纳米药物临床转化率低的瓶颈问题,本课题组从高效、智能药物递送纳米载体和纳米反应器的开发和纳米-血液蛋白相互作用两方面开展研究。基于胶体化学和界面聚合,开发了纳米乳液原位包覆技术,针对特定药物的具体性质(如亲疏水性、生物活性、分子尺寸、释药需求等),发展出一系列特异性纳米胶囊药物递释载体和酶级联纳米反应器;结合材料表面修饰与表征、蛋白质组学和细胞、动物实验,建立了纳米材料-血液蛋白相互作用的研究方法学,揭示了纳米药物与血液蛋白吸附行为的作用机制、生物效应及调控方法,为全面、深入认识纳米药物的体内命运、推动抗肿瘤纳米药物的临床转化提供了重要研究基础。
| |||||
代表性成果 | |||||
代表性论文(*通讯作者;+共同一作) |
|
|
| ||
31. Z. Wang,S. Jiang,*et al. Integrating Incompatible Tandem Photobiocatalysis in Artificial Cells Enables Metabolic Modulation of Natural Cells,Sci. Adv.2025, 11, eadu4828. 30. W. Han, S. Jiang,* et al. Self-Sustained Biophotocatalytic Nano-Organelle Reactors with Programmable DNA Switches for Combating Tumor Metastasis. Adv. Mater.2025, 37 (9), 2415030. 29. F. Fu,+ X. Hu,+S. Jiang,* et al. Engineering Artificial Mitochondria with Self-Amplifying ProtonGeneration for Autonomous Energy Supply and MetabolicCoupling in Artificial Cells, Angew. Chem. 2025, e202514980. 28. Y. Ge,+F. Fu,+S. Jiang,* et al. Physiological pH Transition‐Driven Protein Corona Dynamics Regulate Cellular Uptake and Inflammatory Responses of Silica Nanoparticles. Adv. Sci. 2025, e02788. 27. F. Fu, S. Jiang,* et al. In situ characterization techniques of protein corona around nanomaterials. Chem. Soc. Rev.2024,53, 10827. 26. M. Prawatborisut, S. Jiang,*et al.Modulating Protein Corona and Materials-Cell Interactions with TemperatureResponsive Materials. Adv. Funct. Mater.2022, 32, 2106353. 25. R. Wang, S. Jiang,* et al. Liposomal Enzyme Nanoreactors based on Nanoconfinement for Efficient Anti-Tumor Therapy. Angew. Chem. 2023, 62, e202308761. 24. J. Gonçalves, S.Jiang,*et al. Confining the Sol-Gel Reaction at the Water/Oil Interface: Creating Compartmentalized Enzymatic Nano-Organelles for Artificial Cells. Angew. Chem. 2023, 62, e202216966. 23.S.Jiang,K. Landfester,* et al. Synthetic Silica Nano-Organelles for Regulation of Cascade Reactions in Multi-Compartmentalized Systems.Angew. Chem. 2021, 133, 2. 22. S. Jiang,K. Landfester,*et al.Synergistic Anticancer Therapy by Ovalbumin Encapsulation-Enabled Tandem ROS Generation.Angew. Chem.2020, 59, 2. 21. W. Huang,+N. Huber,+S. Jiang,+ K.Zhang,* et al. Covalent triazine framework nanoparticles via size-controllable confinement synthesis for enhanced visible light photoredox catalysis. Angew. Chem.2020, 59, 18368-18373. 20. S. Jiang,K. Landfester,*et al. One-Step Preparation of Fuel-Containing Anisotropic Nanocapsules with Stimuli-Regulated Propulsion. ACS Nano2019, 14, 498. 19. Y. Niu, S. Jiang,* et al. In Situ Measurement of Nanoparticle–Blood Protein Adsorption and Its Heterogeneity with Single-Nanoparticle Resolution via Dual Fluorescence Quantification. Nano Lett.2024, 24, 9202. 18. M. Li, S.Jiang,*et al. Brush Conformation of Polyethylene Glycol Determines the Stealth Effect of Nanocarriers in the Low Protein Adsorption Regime. Nano Lett. 2021, 21, 1591. 17. S. Jiang, D. Crespy,* et al. Nanocontainers in and onto Nanofibers. Acc. Chem. Res.2016, 49, 816. 16. M. Li,S. Jiang,*et al. Encapsulation of polyprodrugs enables an efficient and controlled release of dexamethasone. Nanoscale Horiz. 2021, 6, 791-800. 15. S.Jiang, K.Landfester,*et al. Visible light active nanofibrous membrane for antibacterial wound dressing. Nanoscale Horiz.2018,3, 439-446. 14. S.Jiang,D. Crespy,*et al. Efficient Nanofibrous Membranes for Antibacterial Wound Dressing and UV Protection. ACS Appl. Mater. Interfaces 2016,8, 29915-29922. 13. S.Jiang, K.Landfester,*et al. Controlling protein interactions in blood for effective liver immunosuppressive therapy by silica nanocapsules. Nanoscale2020,12, 2626-2637. 12. S.Jiang,D. Crespy,*et al. Tailoring nanoarchitectonics to control the release profile of payloads. Nanoscale2016,8, 11511-11517. 11. S. Jiang,Q. Li,*et al. Effect of surface silanization of carbon fiber on mechanical properties of carbon fiber reinforced polyurethane composites. Compos. Sci. Technol.2015,110, 87-94. 10. S. Jiang,Q. Li,*et al.Multiscale graphene oxide-carbon fiber reinforcements for advanced polyurethane composites. Compos. Part A2016,87, 1-9. 9. S.Jiang, K.Landfester,*et al. Versatile Preparation of Silica Nanocapsules for Biomedical Applications. Part. Part. Syst. Char.2020, 1900484. 8. S.Jiang,D. Crespy,*et al. Directed Assembly of Soft Anisotropic Nanoparticles by Colloid Electrospinning. Macromol. Rapid Commun.2016,37, 1598-1602. 7. S.Jiang,D. Crespy,* S.Mylon,* et al. The structure of fibers produced by colloid-electrospinning depends on the aggregation state of particles in the electrospinning feed. Polymer 2017,127, 101-105. 6. S.Jiang,D. Crespy,*et al. Nanofibrous photocatalysts from electrospun nanocapsules. Nanotechnology2017,28, 405601. 5. S.Jiang,D. Crespy,*et al. Control of the release of functional payloads from redox-responsive nanocapsules. RSC Adv. 2016,6, 104330-104337. 4. S.Jiang,D. Crespy,*et al. Dual-Responsive Multicompartment Nanofibers for Controlled Release of Payloads. RSC Adv. 2016,6, 43767-43770. 3. S.Jiang,Q. Li,*et al. Effect of carbon fiber-graphene oxide multiscale reinforcements on the thermos-mechanical properties of polyurethane elastomer. Polym. Composite2019, 40, E953-E961. 2. R.Thiramanas,+S.Jiang,+V. Mailander, et al. Silica Nanocapsules with Different Sizes and Physicochemical Properties as Suitable Nanocarriers for Uptake in T-cells. Int. J. Nanomedicine2020,15, 6069-6084. 1. Z. He,+S. Jiang,+Q. Li,*et al. Self-healing isocyanate microcapsules for efficient restoration of fracture damage of polyurethane and epoxy resins. J. Mater. Sci.2019, 54, 8262.
| |||||
项目课题 | |||||
1. 国家自然科学基金青年基金,2023-2025,主持。 2. 山东省“泰山学者”青年专家,2026-2029,主持。 3. 山东省重点研发子课题,2025-2027,主持。 4. 山东省优秀青年科学基金项目(海外),2022-2025,在研,主持。 5. 中国海洋大学青年英才工程第一层次基金,2021-2025,在研,主持。 6. 青岛海洋科学与技术国家实验室开放基金,2021-2025,在研,主持。 7. 胶类中药联合创新中心项目,2024-2026,在研,主持。 | |||||